Preclinical development of a nanomedicine candidate for Fabry rare disease treatment to enter clinical phase
Project funded under
The European Innovation Council (EIC)
The European Innovation Council (EIC)

NanoGLA
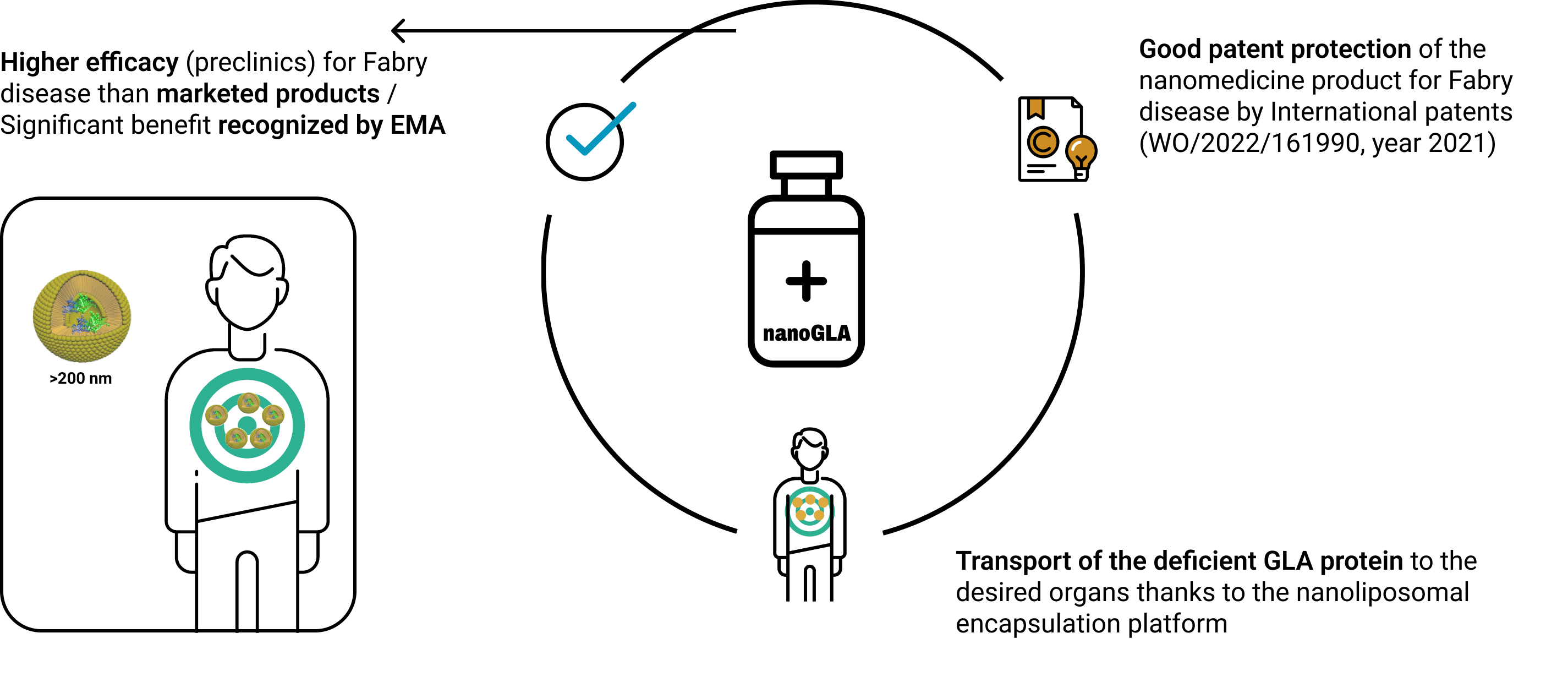
NanoGLA is a patented protected nanopharmaceutical product for the treatment of Fabry disease. It was designated as Orphan Medicinal Product by the EMA in 2021.
Platform
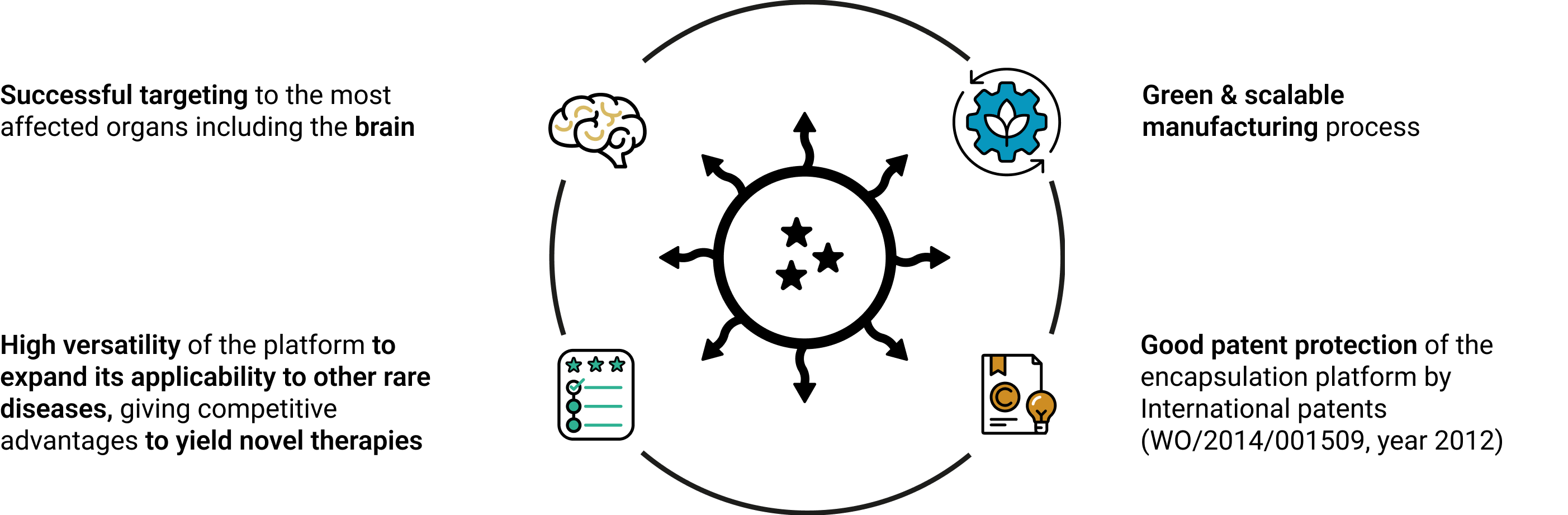
The liposomal platform offers high versatility to expand its applicability to other rare diseases, giving competitive advantages to yield novel therapies.

From bench to bedside
Bring the nanoGLA towards the market and the commercialisation of the patent protected nanoencapsulation platform to generate new product candidates for the treatment of rare diseases.
Months
30
Overall Budget M€
2.5

Lysosomal Storage Disorders - Rare Diseases


Fabry

Pompe
Gaucher

Hunter

Sanfilippo
Fabry Disease

Cause
Fabry disease is caused by a defficiency of the GLA (α-galactosidase-A) protein/enzyme

Current treatment
Enzyme replacement as main therapy of Fabry disease, but:
- low efficiency
- not accessible to brain
- need of frequent dosing
- poor biodistribution profile
Symptoms and nanoGLA treatment
Smart4Fabry EU funded project was the previous project devoted to design the nanoGLA l treatment for the Fabry disease. Herein, a video, prepared during this project, to disseminate the Fabry disease.
Project
Work Plan
In total, six work packages (WPs) will work together to ensure the success of the project during the two years that the Nano4Rare project will be active

Preclinical development
This WP will validate the methods used in the project and the toxicological profile of nanoGLA.
First in human clinical trial design
A specific study plan for the first phase 1 clinical trials must be prepared

Regulatory aspects and ethics
The regulatory and ethical aspects of the first-in-human clinical trials must be addressed in this WP

Business development
A business plan will be prepared for the spin-off that must be incorporated

Dissemination & communication
The Nano4Rare project must be made visible and its results disseminated to the targeted stakeholders.

Project and data management
Resources need to be optimised in order to link them with the other WPs and the European Comission
Nano4Rare Partners
The Institute of Materials Science of Barcelona (ICMAB-CSIC) is a Spanish research center of the Spanish National Research Council (CSIC) specialized in the development of advanced materials for a wide range of applications. Within the institute, the Nanomol-bio group focuses on designing and developing organic materials for biomedical applications, such as nanosystems for drug delivery and release.
People










Collaborators and services suppliers
Nanomol Technologies
Industrial nanoencapsulation manufacturing. Business partner, and EU Phoenix OITB Partner.
Vall d'Hebron
Non-GLP preclinical testing (in vitro and in vivo) and clinial advice on pharmacologic testing.
MyBiotech
CDMO that offers clean room facilities for nanomedicine production and EU Phoenix OITB co-coordinator.
Leanbio
CDMO for the development and manufacturing of therapeutic enzymes and EU Phoenix OITB partner.
Unilabs York Bioanalytical Solutions
Specialists in developing analytical methods for detecting biomolecules.
SPARTA Biodiscovery
A spin-out company that uses SPARTA® technology to characterise the addition of molecules to formulations.
KYMOS group
Photoirradiation studies and bioanalytical services of nanoformulations.
BioNanoNet
A non-profit research organisation that provides toxicological and regulatory advice. EU Phoenix OITB partner.
Luxembourg Institute of Science and Technology
Analysis of biomarkers of the Fabry disease. EU Phoenix OITB coordinator.
Creatio
CDMO specialised in developing advanced therapies.
Anglès Buxeda Advocats
A law firm advising clients in the prevention and resolution of commercial and civil disputes.
Pellisé Abogados
A law firm specialized in intellectual property, competition law, and complex litigation.
Related publications
Authors: Judit Tomsen-Melero, Marc Moltó-Abad, Josep Merlo-Mas, Zamira V. Díaz-Riascos, Edgar Cristóbal-Lecina, Andreu Soldevila, Thomas Altendorfer-Kroath, Dganit Danino, Inbal Ionita, Jan Skov Pedersen, Lyndsey Snelling, Hazel Clay, Aida Carreño, José L. Corchero, Daniel Pulido, Josefina Casas, Jaume Veciana, Simó Schwartz Jr., Santi Sala, Albert Font, Thomas Birngruber, Miriam Royo, Alba Córdoba, Nora Ventosa, Ibane Abasolo, and Elisabet González-Mira
Journal: Science Advances (2024), 10:50
Authors: Judit Tomsen-Melero, Josep Merlo-Mas, Aida Carreño, Santi Sala, Alba Córdoba, Jaume Veciana, Elisabet González-Mira, and Nora Ventosa
Journal: Advanced Drug Delivery Reviews 190 (2022) 114531
Authors: Judit Tomsen-Melero,
Solène Passemard, Natalia García-Aranda, Zamira Vanessa Díaz-Riascos, Ramon González-Rioja, Jannik Nedergaard Pedersen, Jeppe Lyngsø, Josep Merlo-Mas, Edgar Cristóbal-Lecina, José Luis Corchero, Daniel Pulido, Patricia Cámara-Sánchez, Irina Portnaya, Inbal Ionita, Simó Schwartz Jr., Jaume Veciana, Santi Sala, Miriam Royo, Alba Córdoba, Dganit Danino, Jan Skov Pedersen, Elisabet González-Mira, Ibane Abasolo, and Nora Ventosa
Journal: ACS Appl. Mater. Interfaces (2021) 13, 7825−7838
Authors: Josep Merlo-Mas, Judit Tomsen-Melero, José-Luis Corchero, Elisabet González-Mira, Albert Font, Jannik N. Pedersen, Natalia García-Aranda, Edgar Cristóbal-Lecina, Marta Alcaina-Hernando, Rosa Mendoza, Elena Garcia-Fruitós, Teresa Lizarraga, Susanne Resch, Christa Schimpel, Andreas Falk, Daniel Pulido, Miriam Royo, Simó Schwartz Jr., Ibane Abasolo, Jan Skov Pedersen, Dganit Danino, Andreu Soldevila, Jaume Veciana, Santi Sala, Nora Ventosa, and Alba Córdoba
Journal: The Journal of Supercritical Fluids (2021), 173,105204
Authors: Ingrid Cabrera, Ibane Abasolo, José L. Corchero, Elisa Elizondo, Pilar Rivera Gil, Evelyn Moreno, Jordi Faraudo, Santi Sala, Dolores Bueno, Elisabet González-Mira, Merche Rivas, Marta Melgarejo, Daniel Pulido, Fernando Albericio, Miriam Royo, Antonio Villaverde, Maria F. García-Parajo, Simó Schwartz Jr., Nora Ventosa, and Jaume Veciana
Journal: Adv. Healthcare Mater. (2016), 5, 829–840
Authors: Ingrid Cabrera, Elisa Elizondo, Olga Esteban, José Luis Corchero, Marta Melgarejo, Daniel Pulido, Alba Córdoba, Evelyn Moreno, Ugutz Unzueta, Esther Vazquez, Ibane Abasolo, Simó Schwartz, Jr., Antonio Villaverde, Fernando Albericio, Miriam Royo, Maria F. García-Parajo, Nora Ventosa, and Jaume Veciana
Journal: Nano Lett. (2013), 13, 3766−3774
DOI: 10.1021/nl4017072
Contact
ICMAB
Institut de Ciència de Materials de Barcelona (ICMAB-CSIC)
-
Carrer dels Til·lers s/n, Campus de la UAB, 08193 Bellaterra, Barcelona, Spain
-
This email address is being protected from spambots. You need JavaScript enabled to view it. -
Stay connected and get the latest updates on our project's progress, events, and news! Follow us on LinkedIn to be part of our journey.

This project has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement n°101136772.
Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Executive Agency. Neither the European Union nor the European Research Executive Agency can be held responsible for them.